Periodic Table Blocks S P D F Periodic Table Timeline
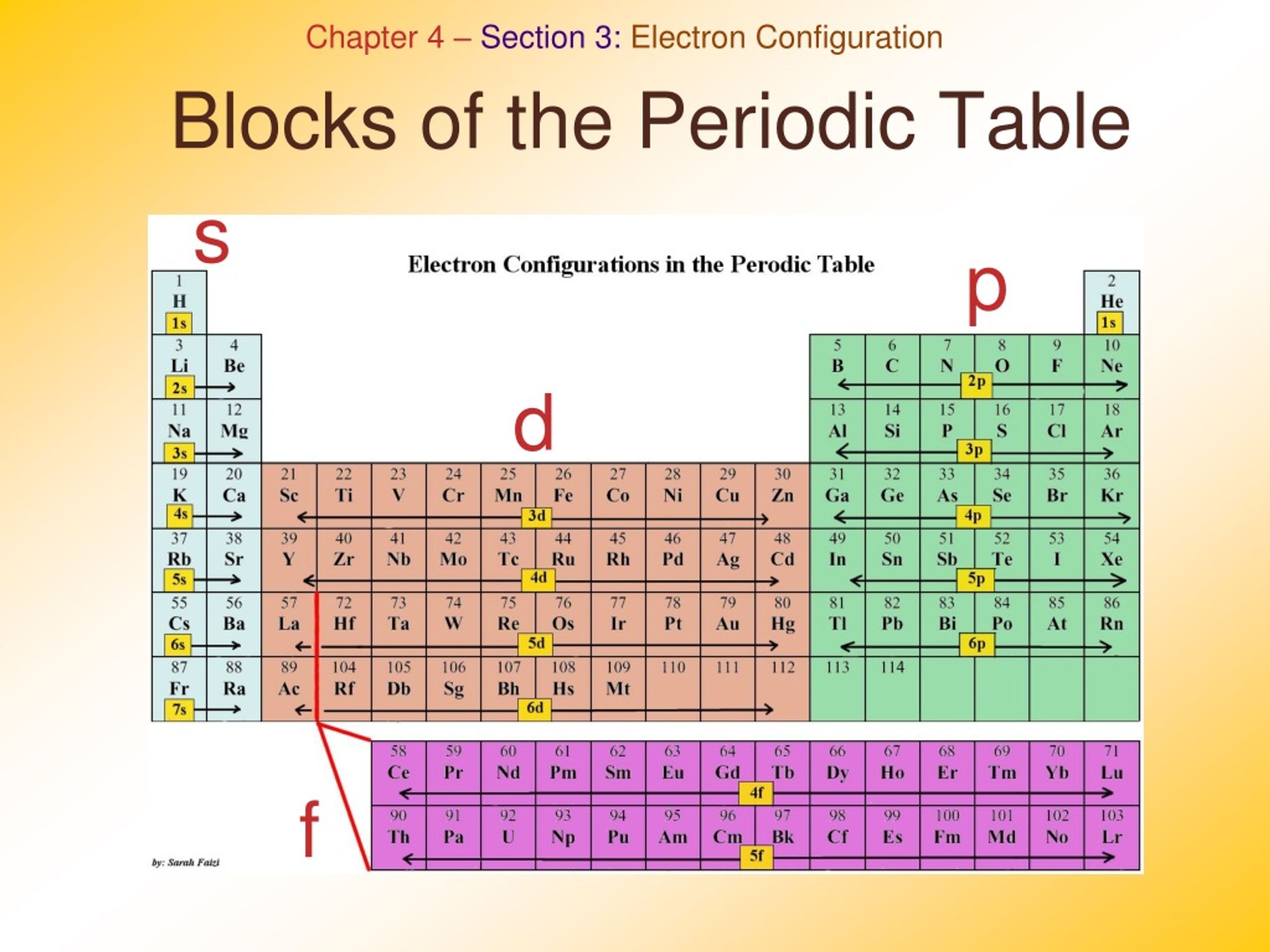
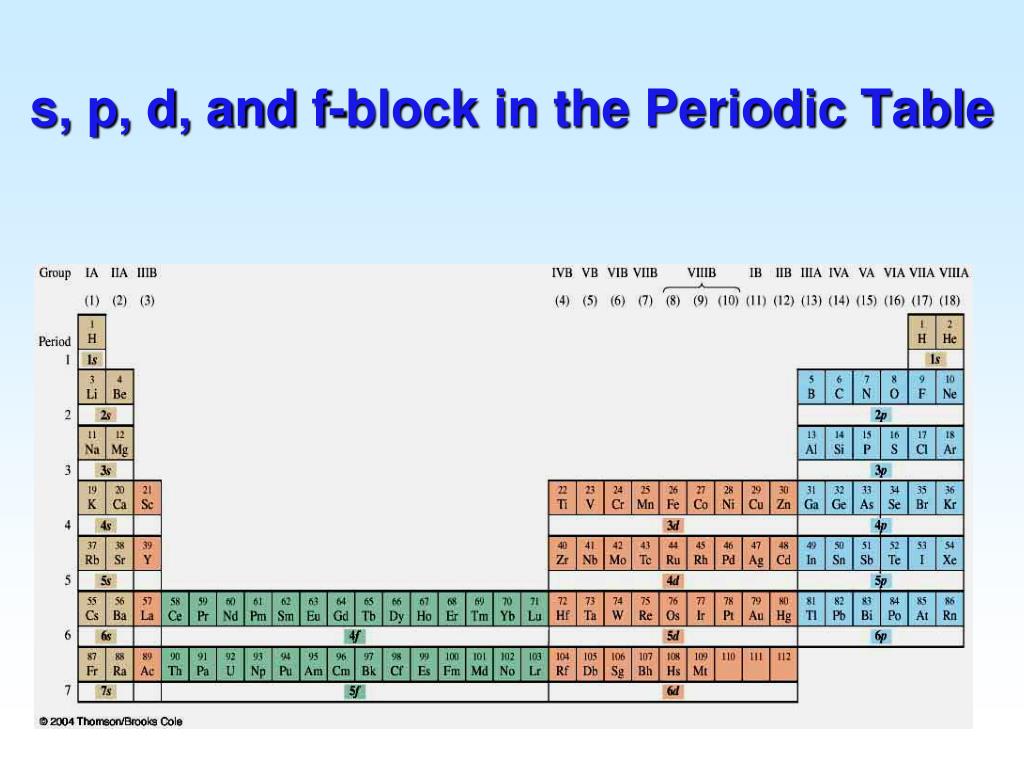
The arrangement of atoms in the periodic table results in blocks corresponding to filling of the. np, nd, and nf orbitals to produce the distinctive chemical properties of the elements in the s block, p block, d block, and f block, respectively. 1.1.9: Electron Configurations and the Periodic Table is shared under a CC BY-NC-SA 4.0 license.
Periodic Table With S P D F Blocks Pdf Periodic Table Timeline
The labels s, p, d and f blocks of the Periodic Table refer to the subshell that is being filled with electrons. ⚛ Group 1 elements occur at the beginning of a new row (Period) of the Periodic Table. The highest energy level (valence shell) contains only 1 electron in an s subshell. ⚛ Group 2 elements occur directly to the right of Group 1.
S P D F Block Elements Periodic Table Periodic Table Timeline
Steps for Identifying S, P, D, & F-Block Elements. Step 1: Find the element on the periodic table. Step 2: Use periodic table landmarks and mnemonic devices to determine the block. Vocabulary for.

Periodic Table Blocks S P D F
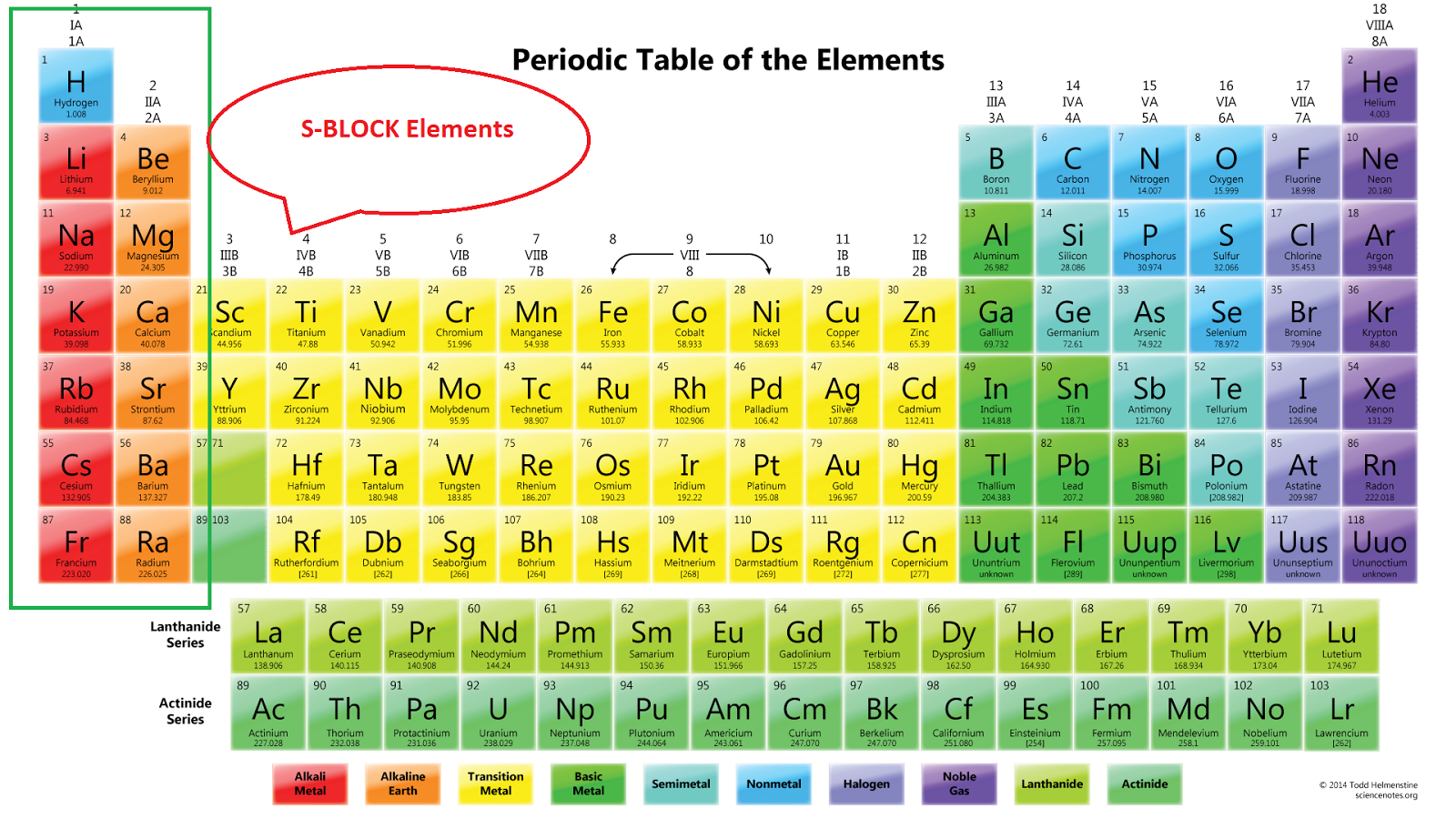
We can classify it into four blocks S - Block Elements. General Electronic configuration is ns 1-2; This block is situated at the extreme left of the periodic table and contains elements of group 1 and 2. Group I elements are known as alkali metals. Group II elements are known as Alkali earth metal. These elements are soft metals.
Periodic Table Blocks S P D F Periodic Table Timeline
A The group 2 elements are in the s block of the periodic table, and as group 2 elements, they all have two. np, nd, and nf orbitals to produce the distinctive chemical properties of the elements in the s block, p block, d block, and f block, respectively. 6.9: Electron Configurations and the Periodic Table is shared under a CC BY-NC-SA 4.

Download Periodic Table Of Elements S P D F Blocks Online Printable PDF DOC
A block of the periodic table is a set of elements unified by the atomic orbitals their valence electrons or vacancies lie in. Each block is named after its.
Electron Configuration of Transition Metals Chemwiki
In this article, we are going to study the similarities and periodicity of elements across the periodic table. We will study the different blocks: s-block, p-block, d-block, f-block and their characteristic properties, metals, metalloids, nonmetals, halogens, and noble gases. Read more about the Position of Hydrogen in Periodic Table, here.

A block diagram of the periodic table shows which sublevels are being filled at any point.
Download scientific diagram | The periodic table of s‐, p‐, d‐, and f‐block elements. from publication: The Pivotal Role of s‐, p‐, and f‐Block Metals in Water Electrolysis: Status.
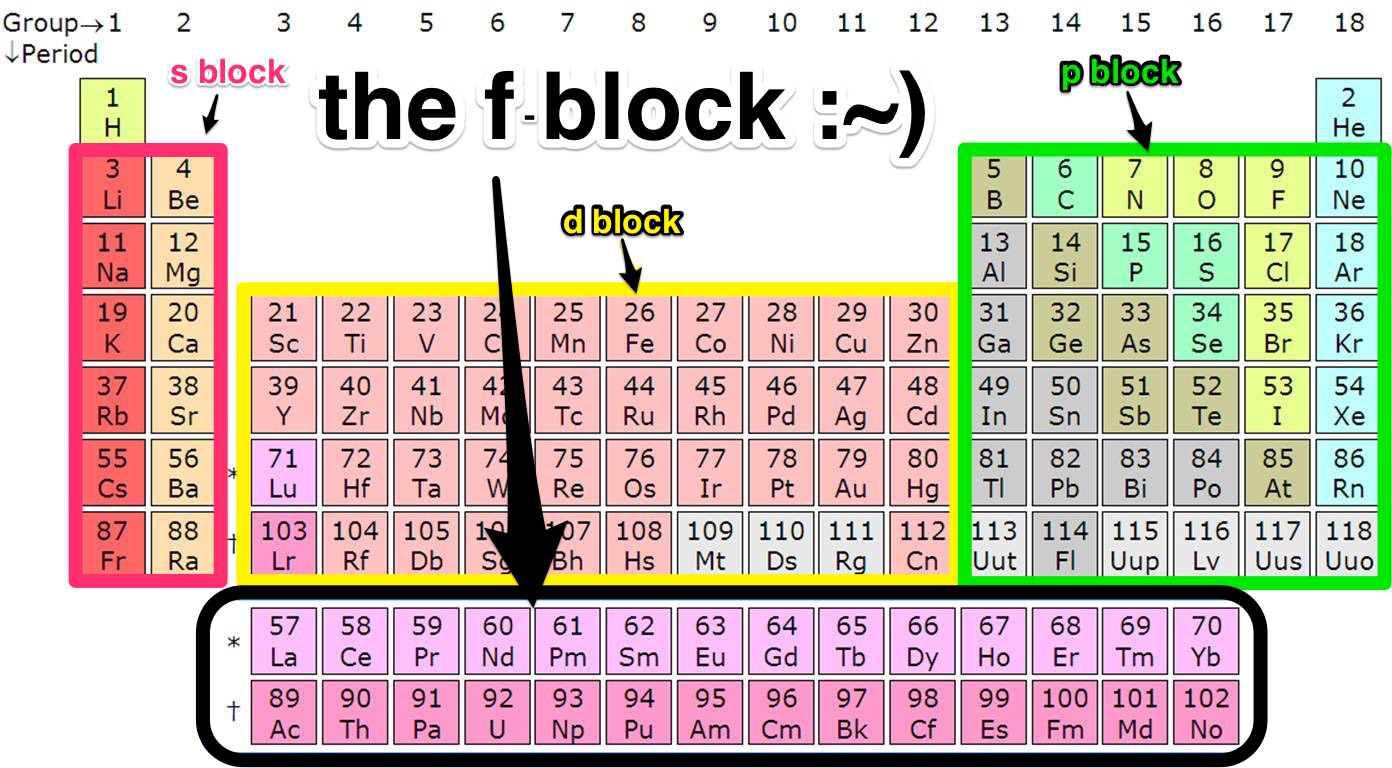
The FBlock An introduction Scienceline
Therefore, we can finally conclude that all the elements exist in their respective blocks, namely s block, p block, d block and f block elements. The S block contains the elements of Group 1 and 2 of the periodic table. D block elements are those elements that can be found in the modern periodic table from the third group to the twelfth group.
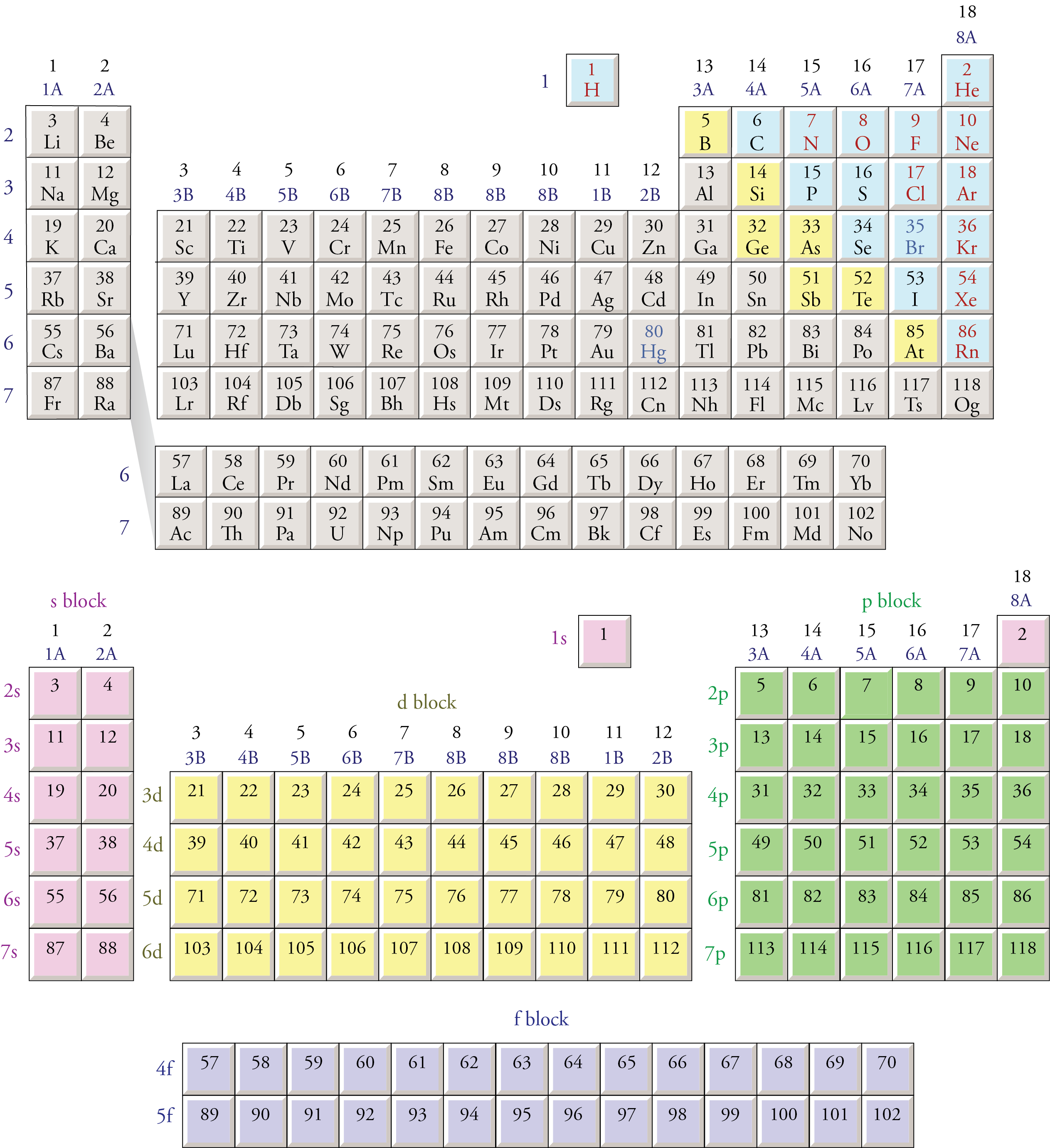
Electron configurations
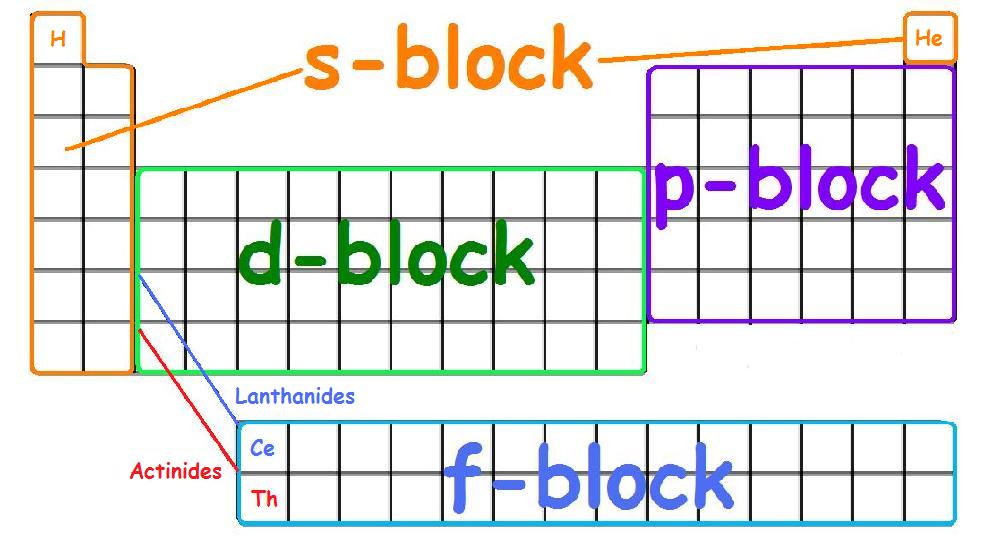
These are s, p, d, and f block elements that constitute the whole periodic table. The term block was used by Charles Janet for the first time when he introduced his left step periodic table (LSPT). The divisions into the blocks are characterized by their distinctive nature.
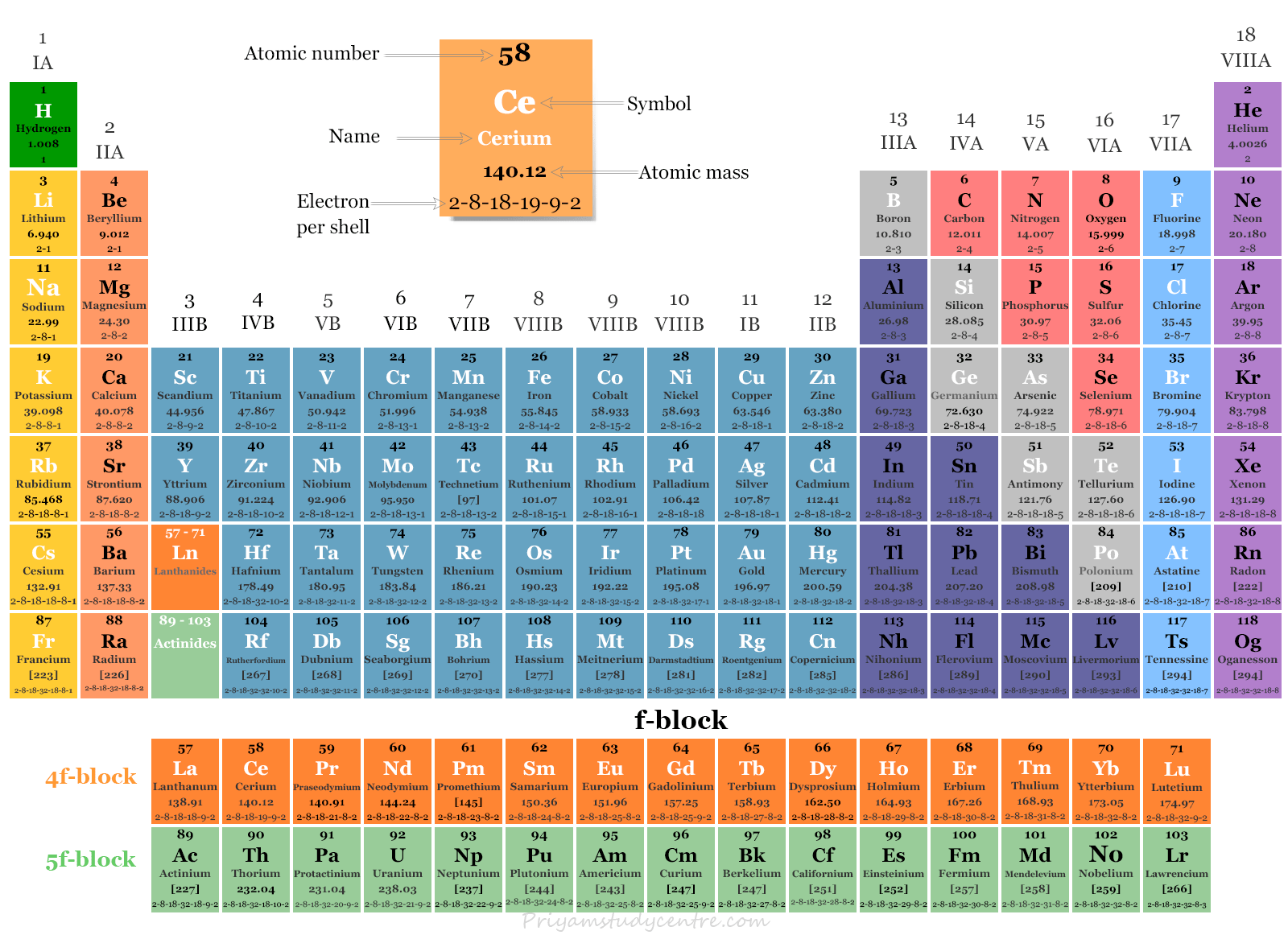
f block Elements Lanthanides and Actinides Periodic Table
The periodic table of elements can be organized by blocks (s, p, d, f, g). Learn what element blocks are and their properties and characteristics.. P-block: P-block elements include the last six element groups of the periodic table, excluding helium. The p-block elements include all of the nonmetals except for hydrogen and helium, the.
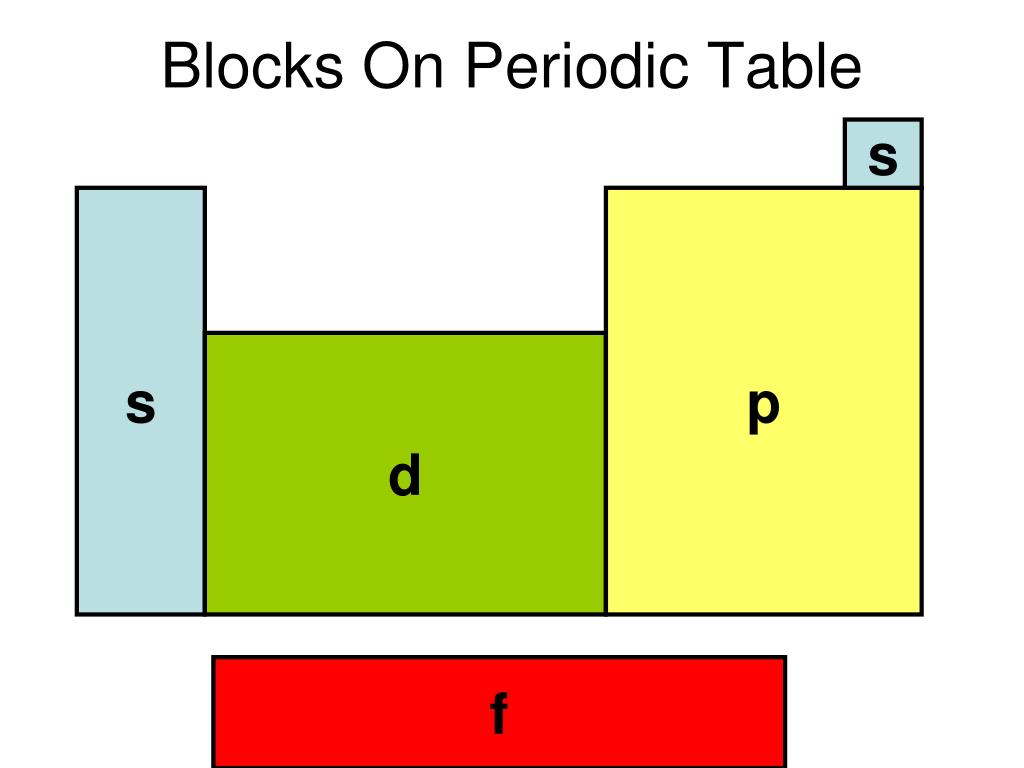
PPT Chapter 5 Electrons In Atoms PowerPoint Presentation, free download ID1755381
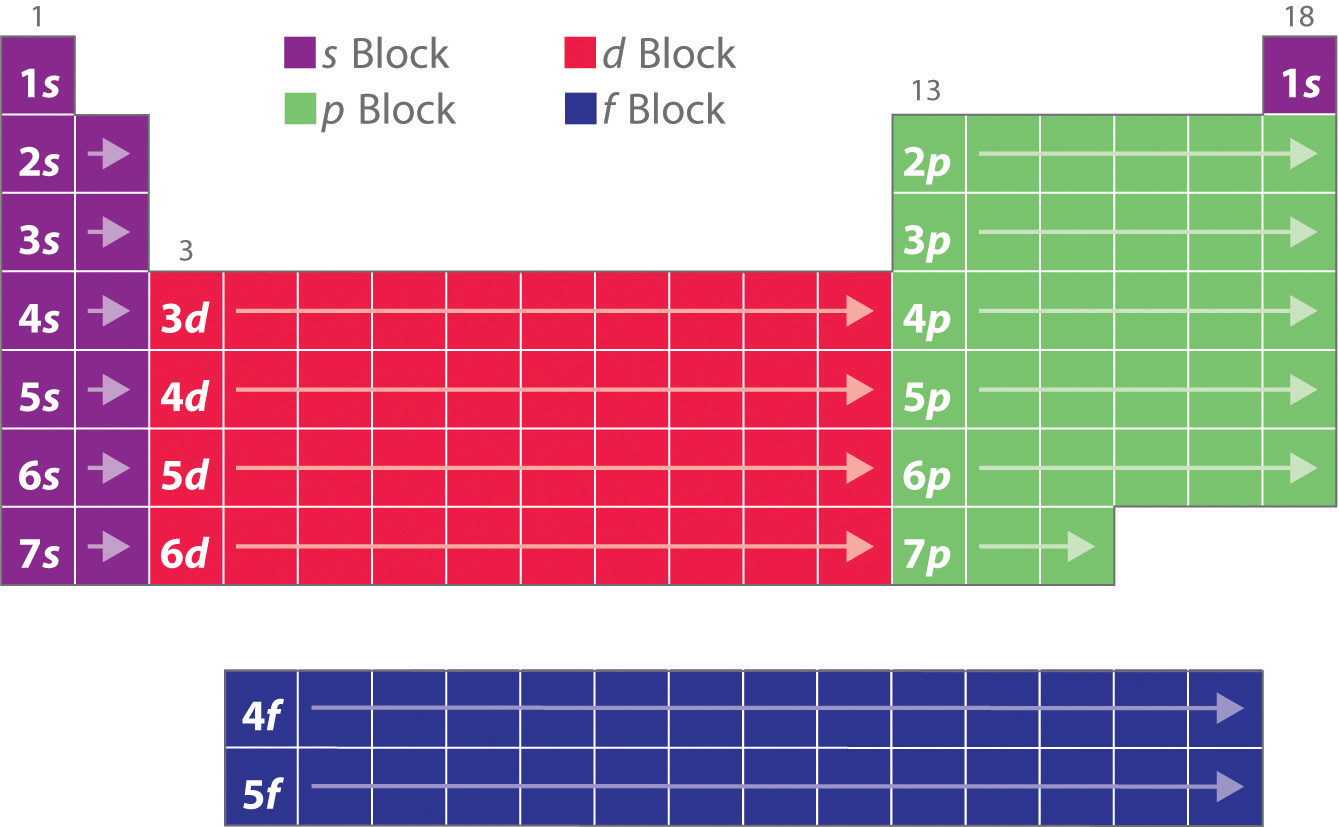
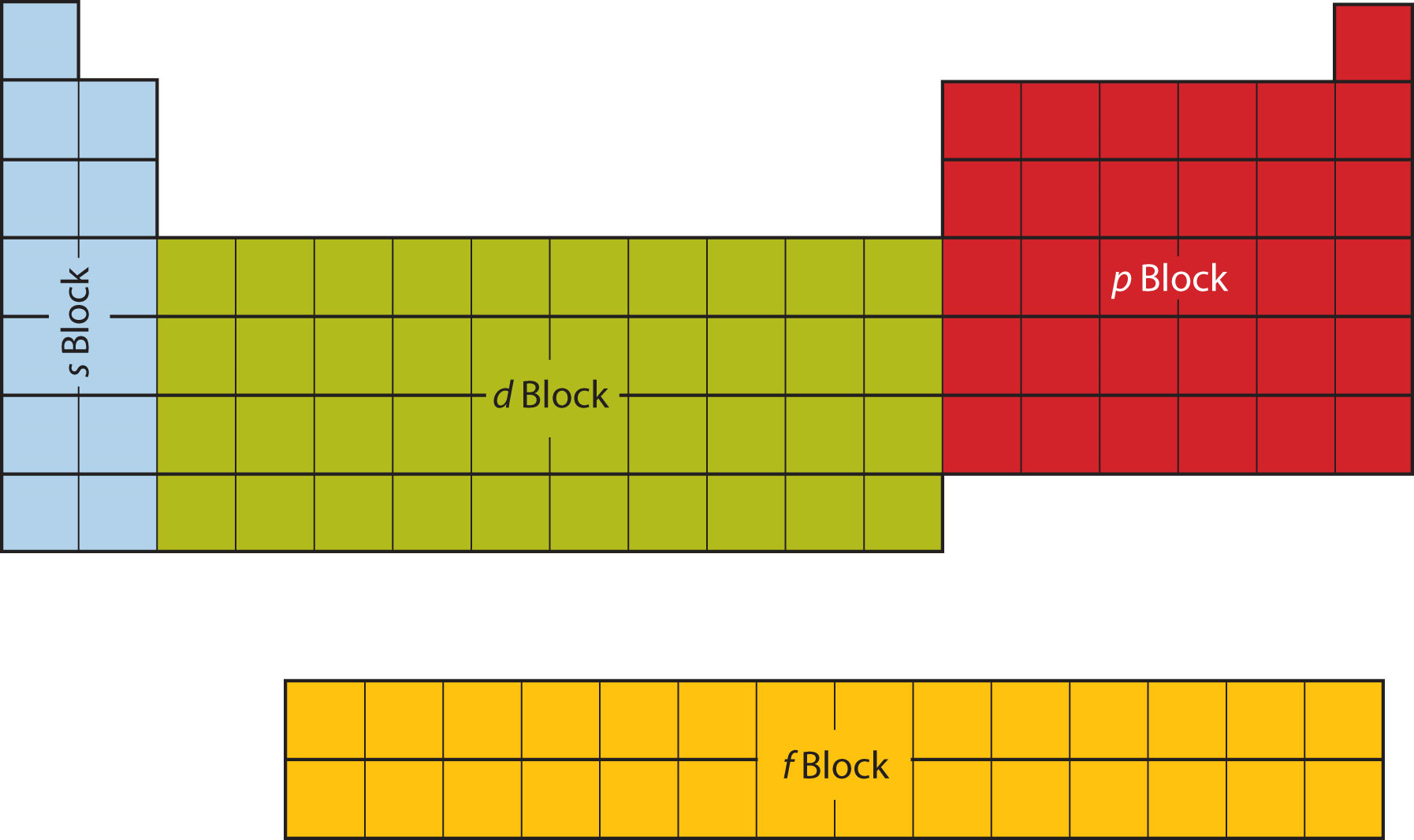
These sublayers being designated by the letters s, p, d, f or even g, the corresponding blocks are designated by these same letters. There are four blocks in the standard periodic table, plus a fifth appearing from the hypothetical 8th period: block s for ℓ = 0; block p for ℓ = 1; block d for ℓ = 2; block f for ℓ = 3;
8.4 Electronic Structure and the Periodic Table Chemistry LibreTexts
A long periodic table showing, from left to right: the s-, d-, f-, and p-blocks. They are named after the orbital. A block on the periodic table is a group of elements that all have their electrons in the same atomic orbital.There are four blocks, s-, d-, f, and p-. The word "block" was first used to describe this by Charles Janet.
PPT Chapter 8 PowerPoint Presentation, free download ID6535306
Properties of f-Block elements: (contain f-electrons in their valence shell). Electronic configuration: n s 2 (n − 1) d (0 − 1) (n − 2) f (1 − 14) 1) f - block elements are also called as inner transition elements. They are: Lanthanides and actinides. 2) Lanthanides. (58Ce- 71Lu ) 3) Actinides are the elements in which the last.

Representative Elements Definition, Examples, Diagrams
A block of the periodic table is a set of elements unified by the atomic orbitals their valence electrons or vacancies lie in. The term seems to have been first used by Charles Janet. Each block is named after its characteristic orbital: s-block, p-block, d-block, f-block and g-block. The block names (s, p, d, and f) are derived from the spectroscopic notation for the value of an electron's.

Modern Periodic Table s,p,d,f Blocks Elements Periodic table blocks, Periodic table, Element
Periodic Table #1 | Introduction | Periods and Groupshttps://youtu.be/EZYtGgND1REClassification of elements and periodicity in properties #2 | Learning of Pe.