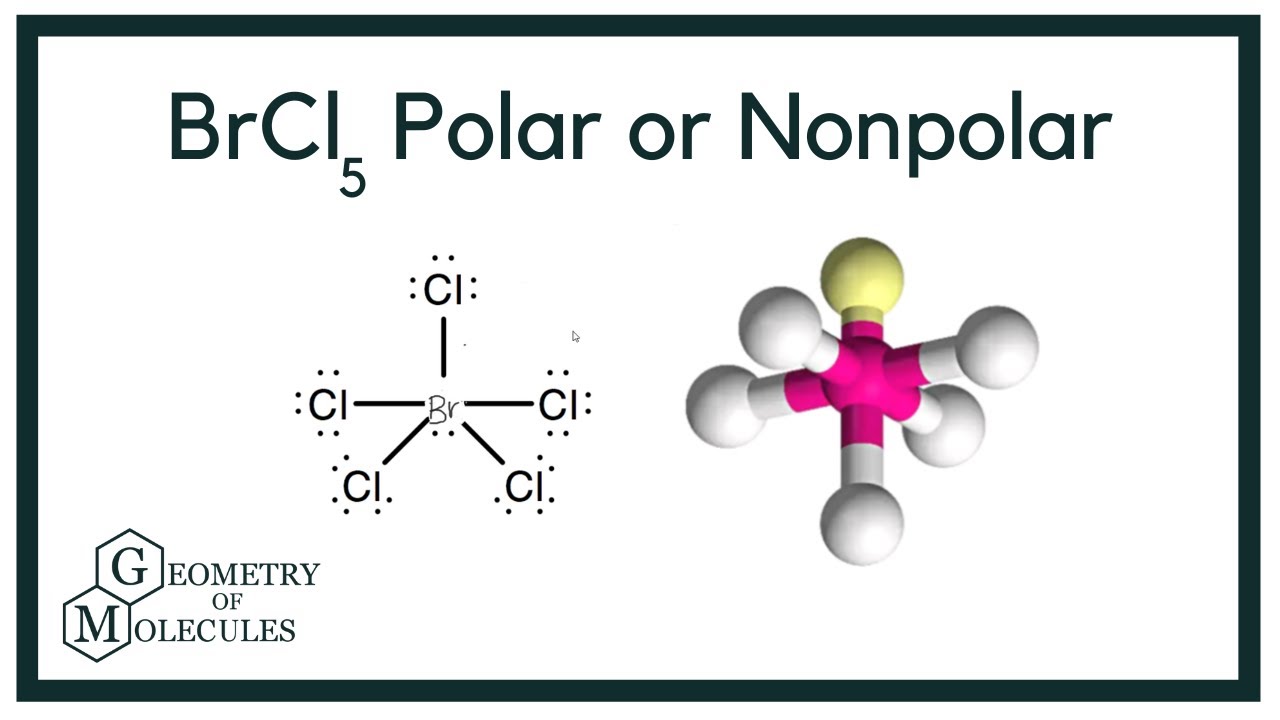
Is BrCl5 Polar or Nonpolar? (Bromine Pentachloride) YouTube
The bond polarity between two atoms can be estimated if you know the electronegativity of both elements. Calculate the electronegativity difference (ΔEN) and average ( EN) of the two electronegativities, and use the table below to determine the bond type and polarity. Calculate the molecular polarity (polar, non-polar) of a chemical bond based.

Is IF5 Polar or Nonpolar? Techiescientist
Guide Is If5 Polar Or Nonpolar September 22, 2022 Webster West The Iodine pentafluoride chemical formula is IF5. Drawing IF5 Lewis Structure is very easy to by using the following method. Here in this post, we described step by step method to construct IF5 Lewis Structure.

Is IF5 Polar or Nonpolar? Techiescientist
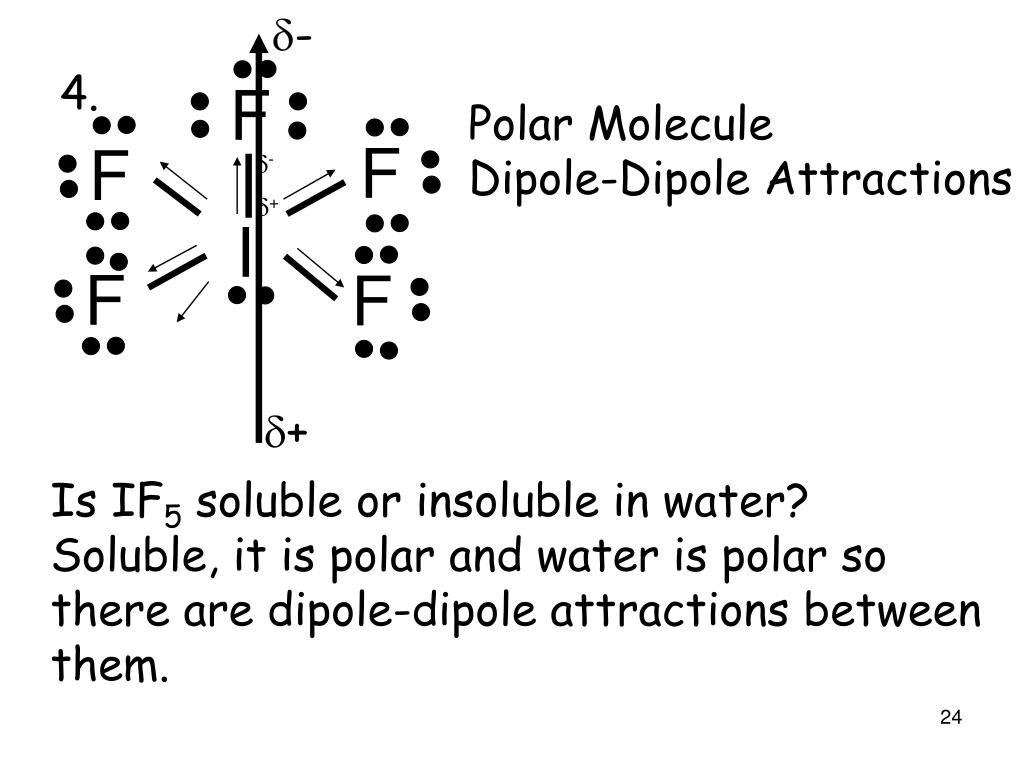
Chemistry Chemistry questions and answers Specify whether the molecule IFs is polar or nonpolar and explain why. The molecule is polar because all the I-F bonds are polar and the net dipole moment is nonzero. The molecule is polar only because all the I-F bonds are polar. The molecule is nonpolar because all the I-F bonds are nonpolar.

Is Hexane Polar or Nonpolar Nehemiahrilrexton
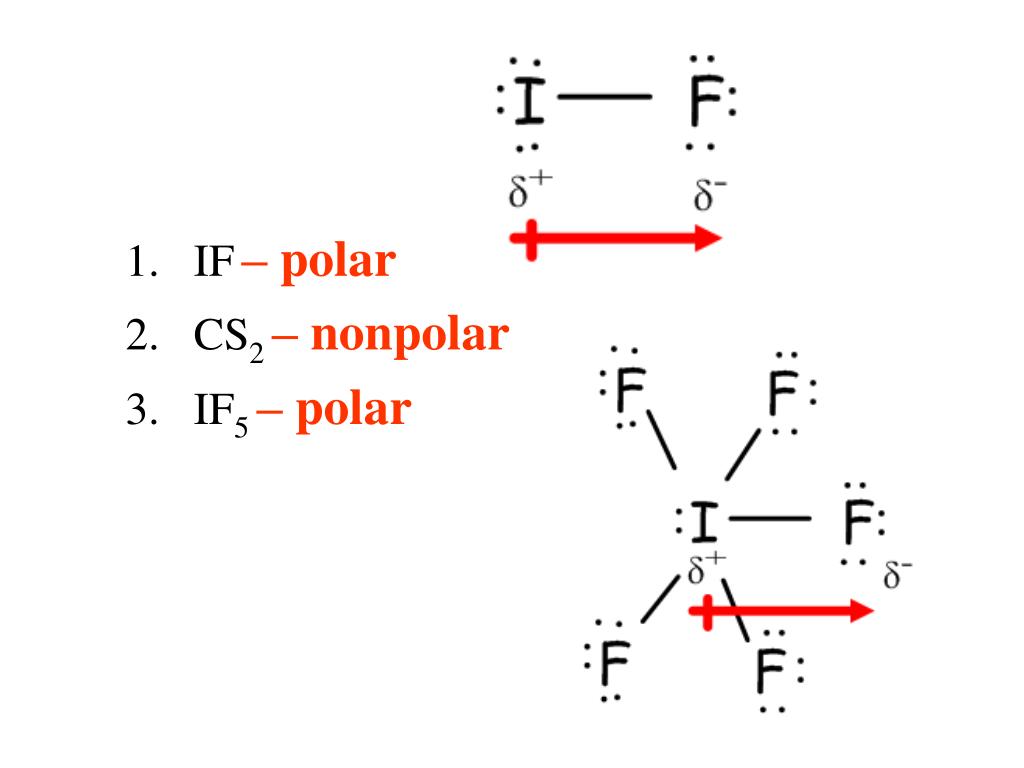
Organic Chemistry 3h 11m. 23. Chemistry of the Nonmetals 1h 51m. 24. Transition Metals and Coordination Compounds 2h 7m. Predict whether each of the following molecules is polar or nonpolar: (a) IF, (b) CS2, (c) SO3, (d) PCl3, (e) SF6, (f) IF5.

Polar Vs Nonpolar Ciencias exatas, Ciência química, Físicoquímica
So, Is IF5 Polar or Nonpolar? IF5 is polar in nature. The molecule has a bent shaped geometrical structure because of lone pair and bond pair repulsion as per VSEPR theory due to which there occurs an imbalance in charge distribution across the molecule.
Cf Polar Or Nonpolar Slidesharetrick My XXX Hot Girl
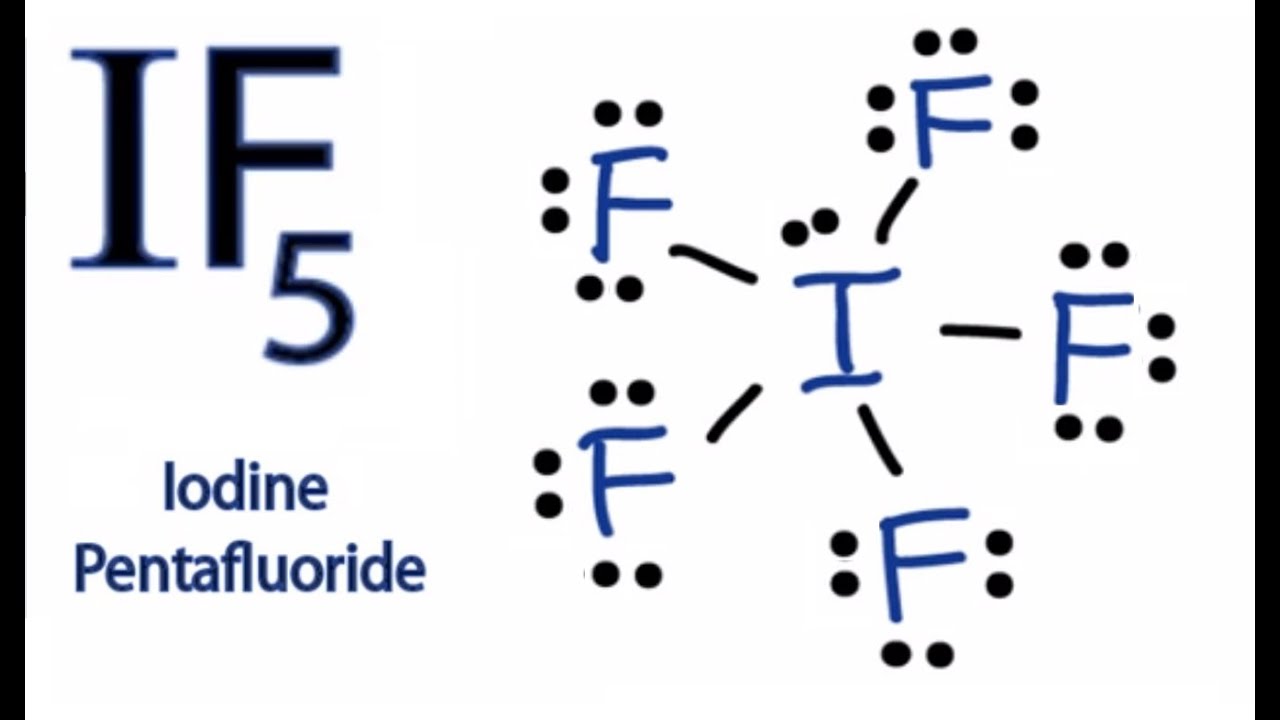
Page Contents show How to draw lewis structure of IF5? The Lewis structure of iodine pentafluoride (IF5) consists of iodine (I) atom at the center. It is bonded to five atoms of fluorine (F) at the sides. There are a total of 6 electron pairs around the central iodine atom in the IF5 lewis dot structure.

Is HF Polar or Nonpolar? (Hydrofluoric Acid) YouTube
The Lewis Structure (Lewis Dot Diagram) for IF5.1. Count electrons2. Put least electronegative atom in centre3. Put one electron pair in each bond4. Fill out.
[Solved] image attached 1. Complete the table below. Indicate whether
1. The first step is to count all the valence electrons of each molecule. In the case of IF5, The Iodine atom has 7 valence electrons. F also has 7 valence electrons. But since there are 5 atoms of F, we multiply 7×5= 35 valence electrons. Adding both we get 35+7= 42. Hence, a total number of valence electrons of IF5= 42.
PPT Part 09 IMFA and Solubility PowerPoint Presentation, free
There are four electron groups around the central atom. As shown in Figure 9.2.2 9.2. 2, repulsions are minimized by placing the groups in the corners of a tetrahedron with bond angles of 109.5°. 3. All electron groups are bonding pairs, so the structure is designated as AX 4.

Polar vs. Nonpolar Bonds — Overview & Examples Expii Ionic Bonding
Henry Agnew (UC Davis) 5.10: Electronegativity and Bond Polarity is shared under a not declared license and was authored, remixed, and/or curated by LibreTexts. Covalent bonds can be nonpolar or polar, depending on the electronegativities of the atoms involved. Covalent bonds can be broken if energy is added to a molecule.
Grade 11 CHAPTER 3 BONDING IN SIMPLE MOLECULES SEMESTER 1
Molecules can be classified as polar or nonpolar. The molecule polar behaves in a different manner as compared to nonpolar. Overview: IF5 Lewis Structure. The central atom is Iodine, which is bordered on five terminals with fluorine atoms( in square pyramidal), and one lone pair on the central in the square pyramidal geometry.
IF5 Lewis Structure How to Draw the Lewis Structure for IF5 สรุป
a. SiCl4 b.. | Channels for Pearson+ Next General Chemistry 13. Liquids, Solids & Intermolecular Forces Molecular Polarity 7:17 minutes Problem 52 Textbook Question Determine whether each molecule is polar or nonpolar. a. SiCl4 b. CF2Cl2 c. SeF6 d. IF5 Verified Solution 7m

Polar and Nonpolar Molecules YouTube
Is IF5 Polar or Nonpolar? Answer: IF5 is a polar molecule due the presence of a lone pair of electrons which due to electron-electron repulsion results in a bent structure. This leads to an unequal distribution of charge within the molecule and therefore a permanent dipole.

Hexano é Polar Ou Apolar AskSchool
Is IF5 polar or non polar? Updated: 8/11/2023 Wiki User ∙ 9y ago Study now See answers (2) Best Answer Copy IF5 is considered a type of polar molecule. It is a polar molecule because it.
PPT Unit 79 Test Review PowerPoint Presentation, free download ID
Iodine pentafluoride (IF5) is a polar molecule. The central iodine (I) atom in IF5 is surrounded by five fluorine (F) atoms forming a square pyramidal shape. The electronegativity of the fluorine (F) atom is greater than the iodine (I) atom.

If5 Lewis Structure
(Explained in 3 Steps) IF5 is a polar molecule because it has poles of partial positive charge (ẟ+) and partial negative charge (ẟ-) on it. Let me explain this to you in 3 steps! Step #1: Draw the lewis structure Here is a skeleton of IF5 lewis structure and it contains five I-F bonds.